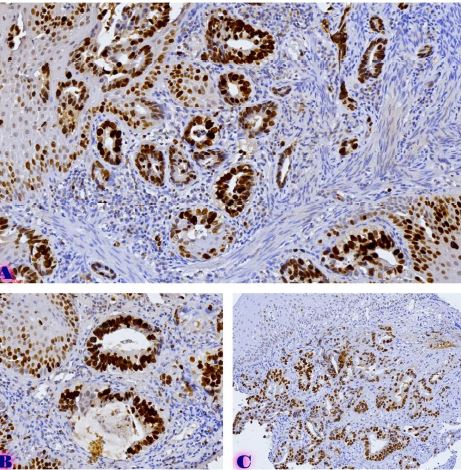
COMPARING CHANGES IN EPITHELIAL-MESENCHYMAL TRANSFORMATION MARKERS IN DIFFERENT TUMOR SITES OF BREAST CANCER WITH HER2-POSITIVE MOLECULAR SUBTYPE
Downloads
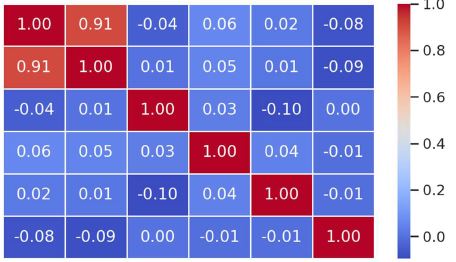
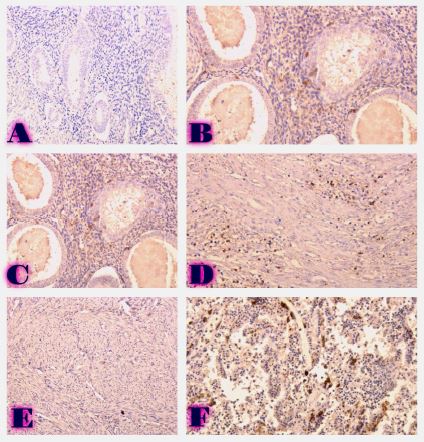
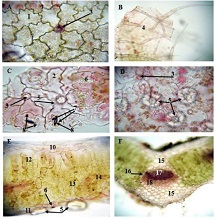
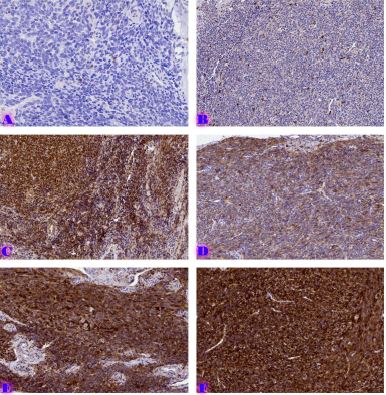
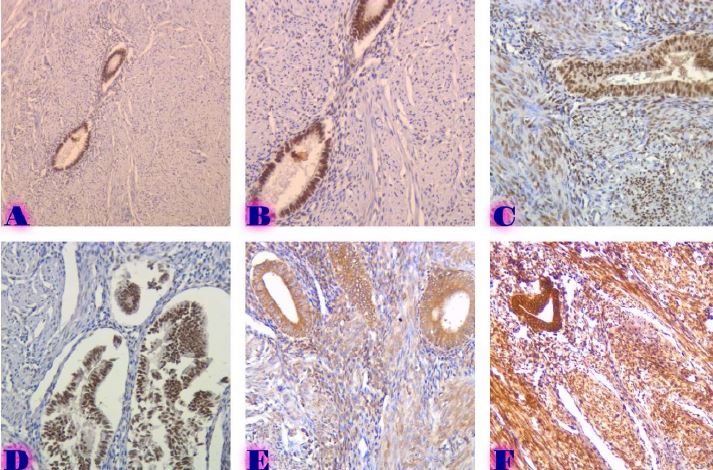
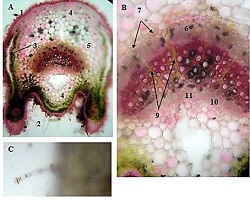
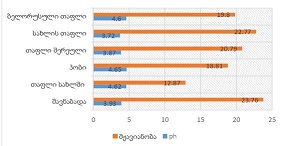
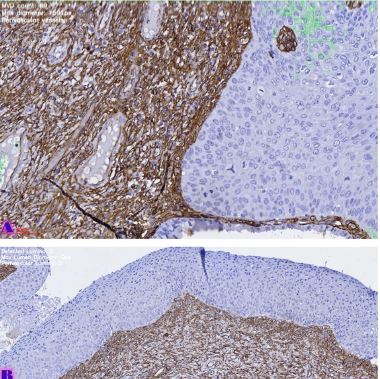
Breast cancer is a heterogeneous disease that can be divided into different molecular subtypes, each with different clinical and pathological characteristics. HER2-positive breast cancer is a subtype characterized by overexpression of the HER2 protein, which is associated with aggressive tumor behavior. The process of epithelial-mesenchymal transformation (EMT) is believed to play a critical role in tumor invasion, metastasis, and resistance to therapy in HER2-positive breast cancer. Tumor-infiltrating lymphocytes (TIL) are an important component of the host's immune response against cancer, and their presence and composition have been shown to correlate with clinical outcome in various types of cancer, including breast cancer. Our study aims to reveal the phenotypic heterogeneity of EMT in different tumor sites, including the primary tumor focus, tumor "Buds' ' and metastatic lymph nodes by molecular typing of HER2-enriched breast cancer. The expression of EMT markers, including E-cadherin, Beta-catenin and vimentin, was measured and significant differences in expression were detected. The presence of EMT in the main tumor focus and tumor "Buds'' was associated with a worse clinical outcome. Furthermore, the presence and composition of TILs varied between tumor sites and correlated with the expression of EMT markers. Based on the findings, it can be assumed that EMT is a heterogeneous process and that the presence of EMT in different tumor sites may have different clinical implications for HER2-positive breast cancer. Identification of biomarkers associated with EMT and TILs may facilitate the development of targeted therapies and improve personalized treatment for patients with HER2-positive breast cancer.
Downloads
B. Nami and Z. Wang, “HER2 in Breast Cancer Stemness: A Negative Feedback Loop towards Trastuzumab Resistance.,” Cancers (Basel), vol. 9, no. 5, Apr. 2017, doi: 10.3390/cancers9050040.
F. Sapna, P. S. S. Athwal, M. Kumar, S. Randhawa, and S. Kahlon, “Therapeutic Strategies for Human Epidermal Receptor-2 Positive Metastatic Breast Cancer: A Literature Review.,” Cureus, vol. 12, no. 8, p. e9522, Aug. 2020, doi: 10.7759/cureus.9522.
M. Gámez-Chiachio, D. Sarrió, and G. Moreno-Bueno, “Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs.,” Cancers (Basel), vol. 14, no. 18, Sep. 2022, doi: 10.3390/cancers14184543.
K. Venetis et al., “HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer”, doi: 10.3389/fmolb.2022.834651.
B. Nami, A. Ghanaeian, C. Black, and Z. Wang, “Epigenetic Silencing of HER2 Expression during Epithelial-Mesenchymal Transition Leads to Trastuzumab Resistance in Breast Cancer,” Life (Basel), vol. 11, no. 9, 2021, doi: 10.3390/LIFE11090868.
Kepuladze Shota, Kokhreidze Irakli, and Burkadze George, “View of THE CHARACTERISTICS OF EPITHELIAL MESENCHYMAL TRANSITION IN DIFFERENT MOLECULAR SUBTYPES OF PRIMARY AND METASTATIC INVASIVE DUCTAL CARCINOMA OF THE BREAST (CRITICAL REVIEW).” https://journals.4science.ge/index.php/jecm/article/view/334/344 (accessed May 14, 2023).
S. Kepuladze, I. Kokhreidze, and G. Burkadze, “Comparative analysis of phenotypic features in the primary tumor, tumour buds and metastatic lymph nodes within Luminal A and Luminal B molecular subtypes of invasive ductal carcinoma of the breast,” GEORGIAN SCIENTISTS, vol. 4, no. 2, pp. 117–140, Apr. 2022, doi: 10.52340/gs.2022.04.02.09.
D. Kajaia, D. Kochiashvili, S. Kepuladze, and G. Burkadze, “Comparative analysis of features of microenvironment, stem cell and epithelial-mesenchymal transformation of non-invasive papillary bladder carcinoma with invasive carcinoma,” GEORGIAN SCIENTISTS, vol. 4, no. 5, pp. 293–303, Dec. 2022, doi: 10.52340/gs.2022.04.05.32.
R. Devadze, A. Gvenetadze, G. Burkadze, and S. Kepuladze, “Features of distribution of intratumoral lymphocytes in ovarian epithelial tumours of different histological types and degree of malignancy,” GEORGIAN SCIENTISTS, vol. 4, no. 5, pp. 383–393, Dec. 2022, doi: 10.52340/gs.2022.04.05.42.
D. Kajaia, D. Kochiashvili, S. Kepuladze, and G. Burkadze, “Comparative analysis of changes in proliferative-apoptotic and hormonal status of non-invasive papillary urothelial carcinomas with molecular subtypes of invasive carcinomas,” GEORGIAN SCIENTISTS, vol. 4, no. 4, pp. 343–361, Sep. 2022, doi: 10.52340//gs.2022.04.04.38.
G. Arveladze, R. Beriashvili, S. Kepuladze, and G. Burkadze, “Problemic issues in Molecular Characterization, Risk of Progression and Recurrence of Basal Cell and Squamous Cell Carcinomas of the Skin,” GEORGIAN SCIENTISTS, vol. 4, no. 5, pp. 86–96, Oct. 2022, doi: 10.52340/gs.2022.04.05.11.
T. Kveliashvili, G. Didava, S. Kepuladze, and G. Burkadze, “Problematic issues of the mucous membrane inflammatory changes of the gallbladder and the features of hormonal expression in the development of dysplasia-carcinoma consequence,” GEORGIAN SCIENTISTS, vol. 4, no. 5, pp. 108–119, Oct. 2022, doi: 10.52340/gs.2022.04.05.13.
N. Qavtaradze, E. (Dodo) Qartvelishvili, S. Kepuladze, and G. Burkadze, “Structural-molecular features of the osteochondral unit of the femur head and the synovial membrane and characteristics of progression and relapse in different types of osteoarthritis,” GEORGIAN SCIENTISTS, vol. 4, no. 5, pp. 175–184, Nov. 2022, doi: 10.52340/gs.2022.04.05.18.
B. Metreveli, D. Gagua, G. Burkadze, and S. Kepuladze, “PROLIFERATIVE CHARACTERISTICS OF EUTOPIC AND ECTOPIC ENDOMETRIUM IN ADENOMYOSIS USING AgNOR TECHNOLOGY,” GEORGIAN SCIENTISTS, vol. 5, no. 1, pp. 59–71, Jan. 2023, doi: 10.52340/gs.2023.05.01.04.
D. B. Doroshow et al., “PD-L1 as a biomarker of response to immune-checkpoint inhibitors,” Nat Rev Clin Oncol, vol. 18, no. 6, pp. 345–362, Jun. 2021, doi: 10.1038/S41571-021-00473-5.
L. Voorwerk et al., “Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial,” Nat. Med., vol. 25, no. 6, pp. 920–928, Jun. 2019, doi: 10.1038/s41591-019-0432-4.
Q. Ma et al., “A PD-L1-targeting chimeric switch receptor enhances efficacy of CAR-T cell for pleural and peritoneal metastasis,” Signal Transduct Target Ther, vol. 7, no. 1, Dec. 2022, doi: 10.1038/s41392-022-01198-2.
Copyright (c) 2023 GEORGIAN SCIENTISTS

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.