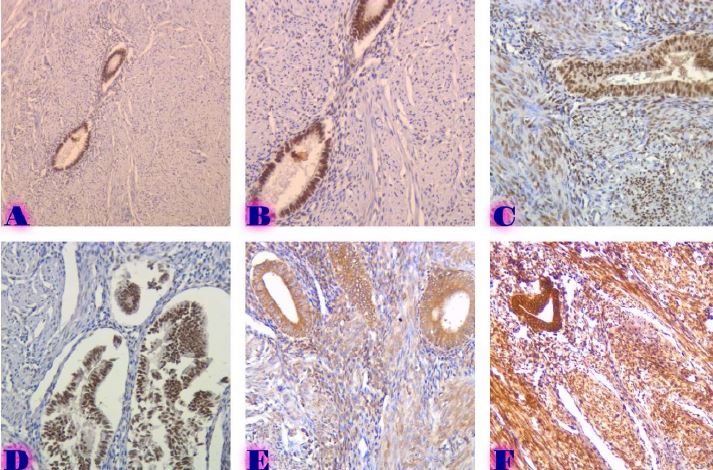
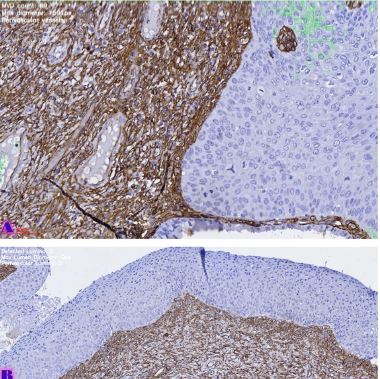
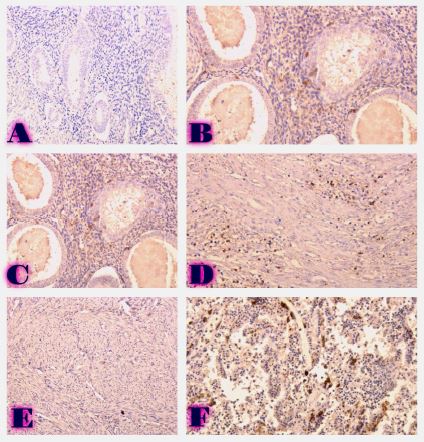
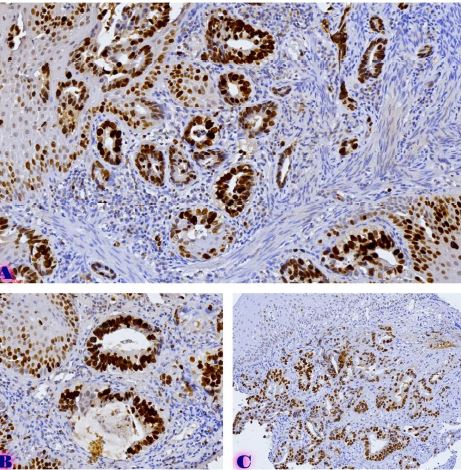
Comparative analysis of changes in proliferative-apoptotic and hormonal status of non-invasive papillary urothelial carcinomas with molecular subtypes of invasive carcinomas
Downloads
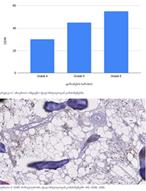
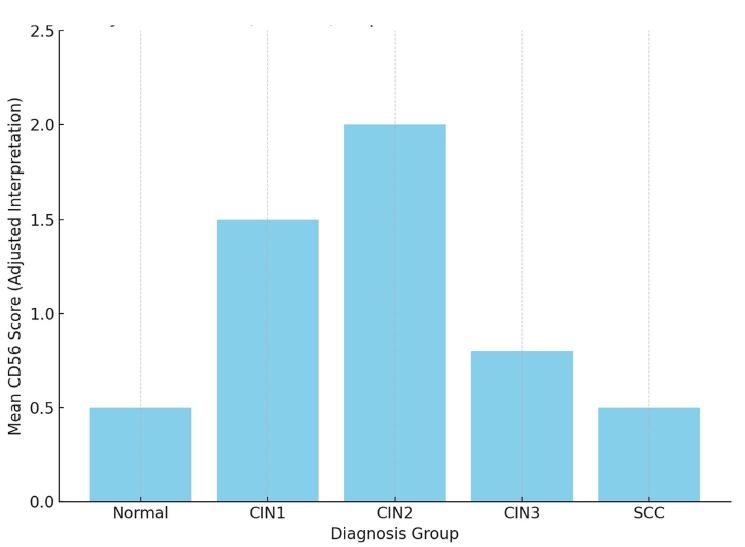
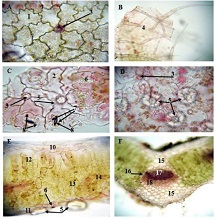
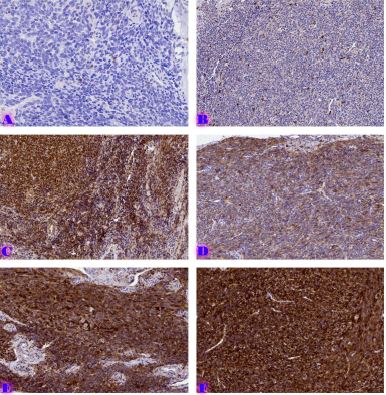
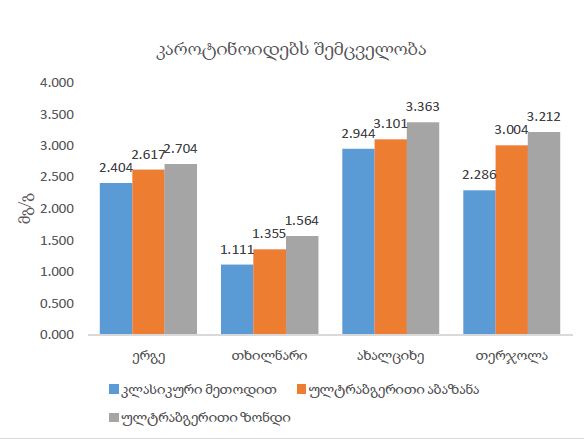
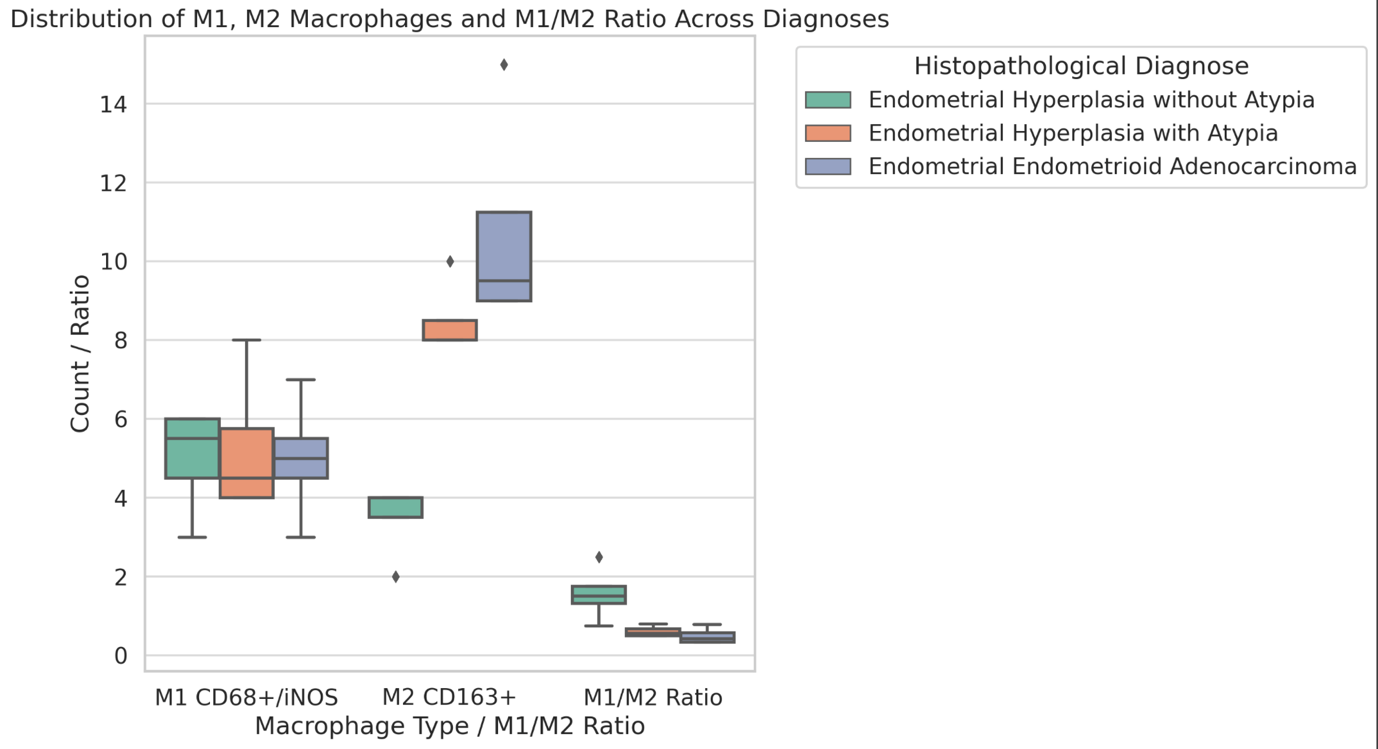
Invasive urothelial carcinoma is the most common bladder malignancy, comprising 90% of all primary bladder carcinomas. According to the statistics of Georgia, according to NCDC data, bladder cancer is the third most common cancer among men. As a result of non-invasive urothelial carcinoma, 70% of the operated patients developed a recurrence in the 5-year period after the surgical intervention on the urinary bladder, among which the share of advanced, metastatic cases is 10-20%.Therefore, regardless of the same degree of differentiation and staging, the recurrence and progression of urothelial carcinoma varies in different patients. A cohort retrograde study was carried out, for which archival material of the teaching-scientific and diagnostic laboratory of Tbilisi State Medical University for the years 2019-2021 was used. The results of the study showed that molecular subtypes of urothelial invasive carcinomas express CK20 and CK5 with different intensity. Moreover, there are mixed, hybrid forms with the coexistence of both markers, which indicates the intratumoral heterogeneous nature of invasive carcinomas, which probably depends on the different clinical course and prognosis of tumors of the same histological type. The phenotypic characteristics of low- and high-grade non-invasive papillary urothelial carcinomas are fundamentally different from the characteristics of invasive urothelial carcinoma, which gives reason to assume that they are different tumors and require additional genomic changes to transform into invasive carcinoma.The phenotypic differences between them are likely to determine the risk of recurrence and invasive transformation. CK20, CK5, apoptotic and proliferative characteristics with androgen receptor expression. Together, it can be used to assess the risk of recurrence and transformation to invasive carcinoma of low- and high-grade noninvasive papillary urothelial carcinomas.
Downloads
A. G. Robertson et al., “Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer,” Cell, vol. 171, no. 3, pp. 540-556.e25, Oct. 2017, doi: 10.1016/J.CELL.2017.09.007.
L. Trilla-Fuertes et al., “Biological molecular layer classification of muscle-invasive bladder cancer opens new treatment opportunities,” BMC Cancer, vol. 19, no. 1, Jun. 2019, doi: 10.1186/S12885-019-5858-Z.
A. Tripathi and E. R. Plimack, “Immunotherapy for Urothelial Carcinoma: Current Evidence and Future Directions,” Curr Urol Rep, vol. 19, no. 12, Dec. 2018, doi: 10.1007/S11934-018-0851-7.
H. Ide, S. Inoue, and H. Miyamoto, “Histopathological and prognostic significance of the expression of sex hormone receptors in bladder cancer: A meta-analysis of immunohistochemical studies,” PLoS One, vol. 12, no. 3, Mar. 2017, doi: 10.1371/JOURNAL.PONE.0174746.
P. A. Humphrey, H. Moch, A. L. Cubilla, T. M. Ulbright, and V. E. Reuter, “The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours,” Eur Urol, vol. 70, no. 1, pp. 106–119, 2016, doi: 10.1016/J.EURURO.2016.02.028.
E. M. Compérat et al., “Grading of Urothelial Carcinoma and The New ‘World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016,’” Eur Urol Focus, vol. 5, no. 3, pp. 457–466, May 2019, doi: 10.1016/J.EUF.2018.01.003.
N. T. Gaisa et al., “Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer,” Virchows Archiv, vol. 458, no. 3, pp. 301–312, Mar. 2011, doi: 10.1007/S00428-010-1017-2.
J. de la ROSETTE, F. SMEDTS, C. SCHOOTS, H. HOEK, and P. LAGUNA, “Changing Patterns of Keratin Expression could be Associated with Functional Maturation of the Developing Human Bladder,” J Urol, pp. 709–717, Aug. 2002, doi: 10.1097/00005392-200208000-00085.
K. T. Mai, A. Busca, and E. C. Belanger, “Flat Intraurothelial Neoplasia Exhibiting Diffuse Immunoreactivity for CD44 and Cytokeratin 5 (Urothelial Stem Cell/Basal Cell Markers): A Variant of Intraurothelial Neoplasia Commonly Associated with Muscle-invasive Urothelial Carcinoma,” Applied Immunohistochemistry and Molecular Morphology, vol. 25, no. 7, pp. 505–512, 2017, doi: 10.1097/PAI.0000000000000334.
S. Jung et al., “The role of immunohistochemistry in the diagnosis of flat urothelial lesions: A study using CK20, CK5/6, P53, Cd138, and Her2/Neu,” Ann Diagn Pathol, vol. 18, no. 1, pp. 27–32, Feb. 2014, doi: 10.1016/J.ANNDIAGPATH.2013.10.006.
D. Sikic et al., “Immunohistochemiocal subtyping using CK20 and CK5 can identify urothelial carcinomas of the upper urinary tract with a poor prognosis,” PLoS One, vol. 12, no. 6, Jun. 2017, doi: 10.1371/JOURNAL.PONE.0179602.
T. Kawahara et al., “Enzalutamide inhibits androgen receptor–positive bladder cancer cell growth,” Urologic Oncology: Seminars and Original Investigations, vol. 34, no. 10, pp. 432.e15-432.e23, Oct. 2016, doi: 10.1016/J.UROLONC.2016.05.016.
T. Powles et al., “Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study,” JAMA Oncol, vol. 3, no. 9, Sep. 2017, doi: 10.1001/JAMAONCOL.2017.2411.
H. D. Pearse, R. R. Reed, and C. v. Hodges, “Radical cystectomy for bladder cancer,” Journal of Urology, vol. 119, no. 2, pp. 216–218, 1978, doi: 10.1016/S0022-5347(17)57437-6.
D. M. Parkin, “The global burden of urinary bladder cancer.,” Scand J Urol Nephrol Suppl, no. 218, pp. 12–20, 2008, doi: 10.1080/03008880802285032.
A. Tripathi and S. Gupta, “Androgen receptor in bladder cancer: A promising therapeutic target,” Asian J Urol, vol. 7, no. 3, p. 284, Jul. 2020, doi: 10.1016/J.AJUR.2020.05.011.
M. Yasui et al., “Androgen receptor mRNA expression is a predictor for recurrence-free survival in non-muscle invasive bladder cancer,” BMC Cancer, vol. 19, no. 1, Apr. 2019, doi: 10.1186/S12885-019-5512-9.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.