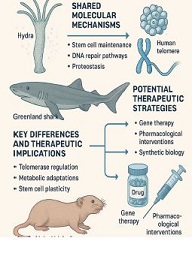
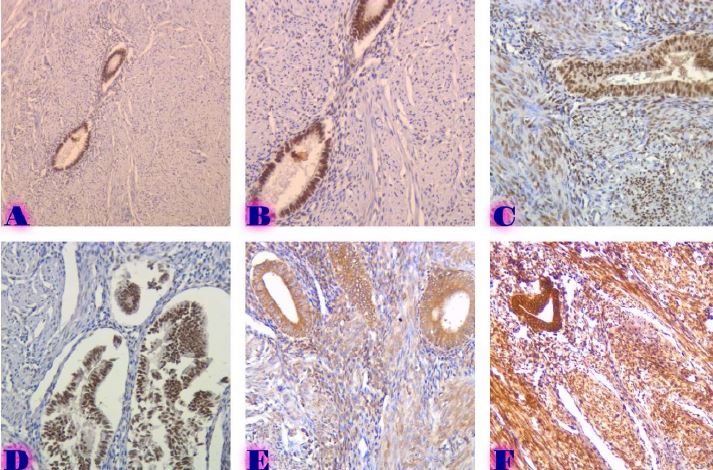
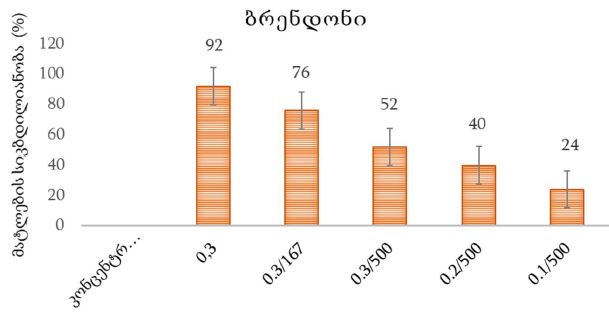
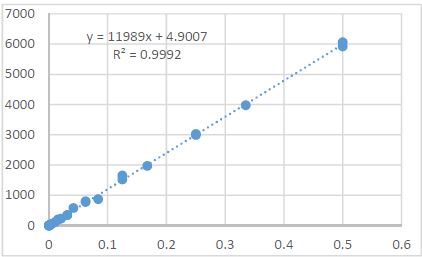
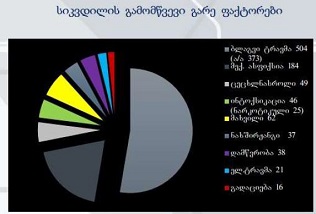
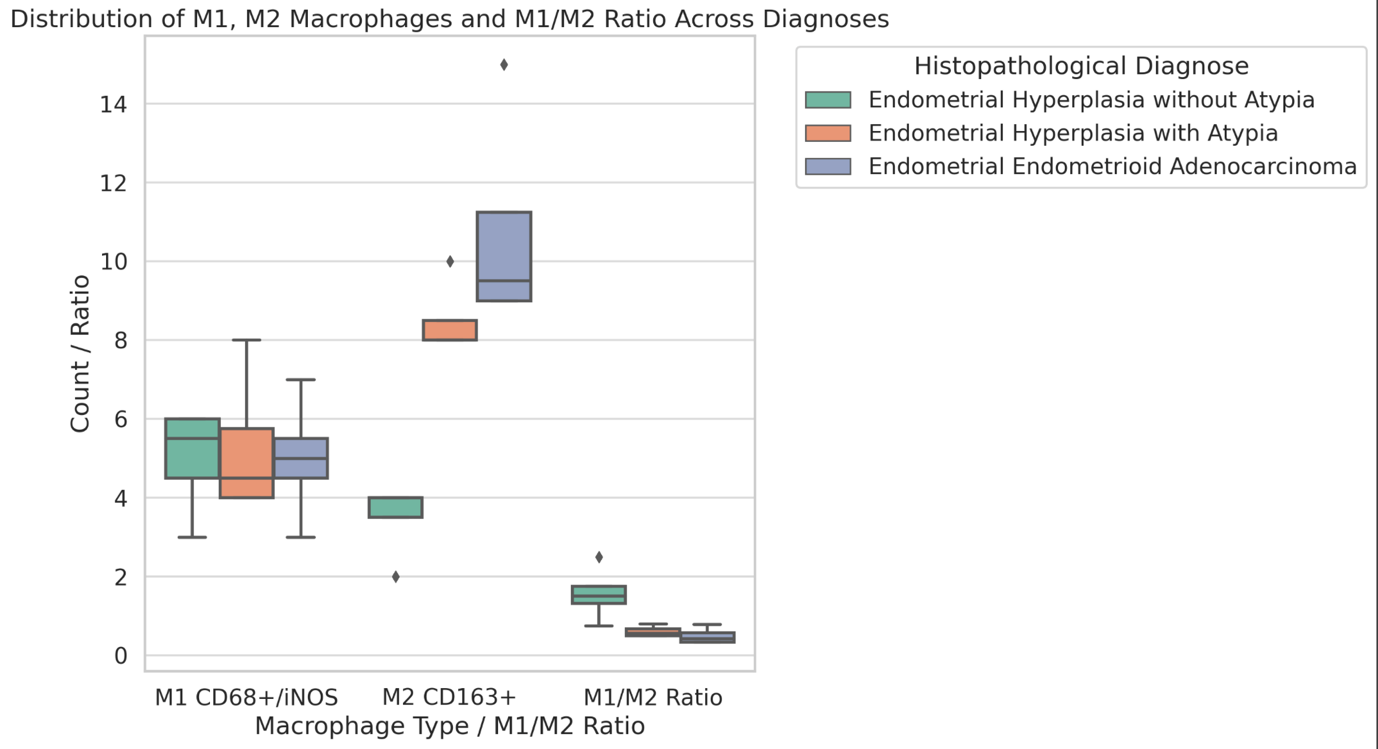
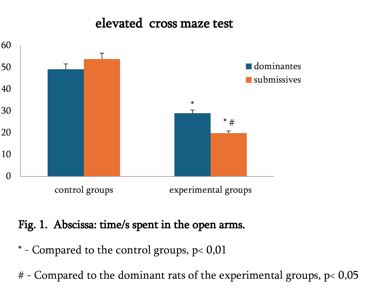
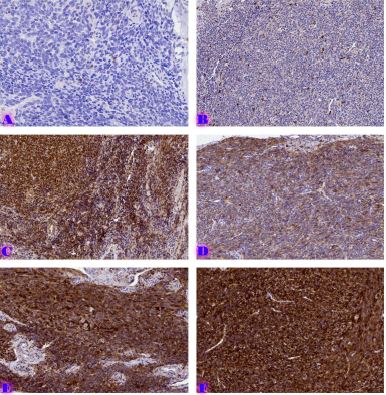
Is the selective accumulation of oldest centrioles in stem cells the main cause of organism ageing?
Downloads
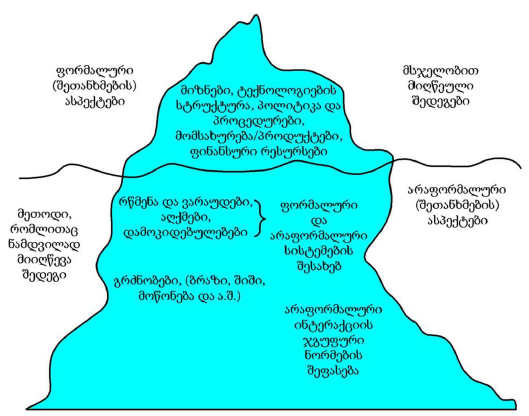
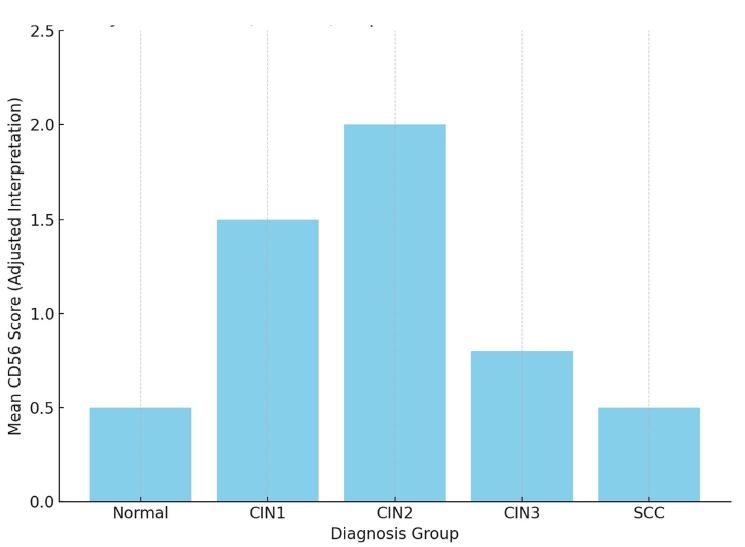
All molecules, structures, cells of organisms are subject to destruction in the process of vital activity. In the organisms of multicellular animals and humans, the process of regeneration is always taking place: the destruction of old cells and their replacement with new ones. Cells are replaced even if the cells are in perfect condition. The earlier the body destroys the matured cells and replaces them with new ones , the younger the body is (the higher the rate of regeneration). Stem cells are the precursor cells of all differentiated cells. Asymmetric division of the mother stem cell gives rise to one, analogue of the mother, daughter cell and another daughter cell that follows a further differentiation pathway. Despite such asymmetric division, the pool of stem cells decreases over time. Moreover, the intervals between stem cell divisions are increasing. The combination of these two processes leads to a decrease in the rate of regeneration and ageing of the organism. During asymmetric division of stem cells, daughter cells, with preserved stem cell potency, selectively retain mother (old) centrioles. Unlike nuclear DNA molecules, repairs do not occur in centrioles. Hypothetically, old centrioles are more susceptible to destruction than other cell structures—making centrioles a potentially structure of the ageing of organisms.
Downloads
Abumuslimov, SS., Nadezhdina, ES., Chentsov, IuS. 1994. [An electron microscopic study of centriole and centrosome morphogenesis in the early development of the mouse]. Tsitologiia.;36(11):1054-61. Russian.
Batty, P., Gerlich, DW. 2019. Mitotic Chromosome Mechanics: How Cells Segregate Their Genome. Trends Cell Biol. doi.org/10.1016/j.tcb.2019.05.007
Bianconi et al. 2013. An estimation of the number of cells in the human body. Ann Hum Biol. doi.org/10.3109/03014460.2013.807878
Binder, MD., Hirokawa, N., Windhorst U. 2009. Cellular potency. (eds) Encyclopedia of Neuroscience. Springer, Berlin, Heidelberg. doi.org/10.1007/978-3-540-29678-2_875
Bobinnec et al. 1998. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. doi.org/10.1083/jcb.143.6.1575
Boldrini et al. 2018. Human Hippocampal Neurogenesis Persists throughout aging. Cell Stem Cell. Doi: 10.1016/j.stem.2018.03.015
Boulan, L., Léopold, P. 2021. What determines organ size during development and regeneration? Development. doi.org/10.1242/dev.196063
Briggs, R., King, TJ. 1952. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs' Eggs. Proc Natl Acad Sci U S A. doi.org/10.1073/pnas.38.5.455
Brown, G., Hughes, PJ., Michell, RH. 2003. Cell differentiation and proliferation--simultaneous but independent? Exp Cell Res. doi.org/10.1016/s0014-4827(03)00393-8
Calarco-Gillam et al. 1983. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. doi.org/10.1016/0092-8674(83)90094-6
Campbel et al. Nuclear transfer in practice. 2001. Cloning Stem Cells. doi.org/10.1089/15362300152725927
Carlson BM, Faulkner JA. 1989. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. doi: 10.1152/ajpcell.1989.256.6. C1262.
Chang, JT., Reiner, SL. 2008. Asymmetric division and stem cell renewal without a permanent niche: lessons from lymphocytes. Cold Spring Harb Symp Quant Biol. doi.org/10.1101/sqb.2008.73.008
Chen, C., Fingerhut, JM., Yamashita, YM. 2016. The ins (ide) and outs (ide) of asymmetric stem cell division. Curr Opin Cell Biol. Doi: 10.1016/j.ceb.2016.06.001
Chen, C., Yamashita, YM. 2021. Centrosome-centric view of asymmetric stem cell division. Open Biol. doi.org/10.1098/rsob.200314
Chichinadze, K. N., & Tkemaladze, D. V. (2008). Centrosomal hypothesis of cellular aging and differentiation. Advances in Gerontology= Uspekhi Gerontologii, 21(3), 367-371. PMID: 19432168
Chichinadze, K., Tkemaladze, D., & Lazarashvili, A. (2012a). New class of RNA and centrosomal hypothesis of cell aging. Advances in Gerontology= Uspekhi Gerontologii, 25(1), 23-28. PMID: 22708440
Chichinadze, K., Tkemaladze, J., & Lazarashvili, A. (2012b). Discovery of centrosomal RNA and centrosomal hypothesis of cellular ageing and differentiation. Nucleosides, Nucleotides and Nucleic Acids, 31(3), 172-183. doi: 10.1080/15257770.2011.648362. PMID: 22356233
Chichinadze, K., Tkemaladze, J., & Lazarashvili, A. (2012c). A new class of RNAs and the centrosomal hypothesis of cell aging. Advances in Gerontology, 2(4), 287-291
Chichinadze, K., Lazarashvili, A., & Tkemaladze, J. (2013). RNA in centrosomes: structure and possible functions. Protoplasma, 250(1), 397-405. doi: 10.1007/s00709-012-0422-6. Epub 2012 Jun 10. PMID: 22684578
Conboy, IM., Rando, TA. 2012. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. Doi: 10.4161/cc.20437
Driesen et al. 2014. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. doi.org/10.1093/cvr/cvt338
Eckfeldt, CE., Mendenhall, EM., Verfaillie, CM. 2005. The molecular repertoire of the 'almighty' stem cell. Nat Rev Mol Cell Biol. Doi: 10.1038/nrm1713
Encinas, JM., Sierra, A. 2012. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. Doi:10.1016/j.bbr.2011.10.010
Ewen-Campen, B., Schwager, EE., Extavour, CG. 2010. The molecular machinery of germ line specification. Mol Reprod Dev. doi.org/10.1002/mrd.21091
Gadde, S., Heald, R. (2004). Mechanisms and molecules of the mitotic spindle. Curr Biol. doi.org/10.1016/j.cub.2004.09.021
Galan et al. 2013. Defining the genomic signature of totipotency and pluripotency during early human development. PLoS One. doi.org/10.1371/journal.pone.0062135
Giehl, KM. 2007. Neuronal development. Prog Exp Tumor Res. doi.org/10.1159/000100041
Gómez et al. 2004. Birth of African Wildcat cloned kittens born from domestic cats. Cloning Stem Cells. doi.org/10.1089/clo.2004.6.247
Gómez et al. 2008. Nuclear transfer of sand cat cells into enucleated domestic cat oocytes is affected by cryopreservation of donor cells. Cloning Stem Cells. doi.org/10.1089/clo.2008.0021
Gönczy, P., Hatzopoulos, GN. 2019. Centriole assembly at a glance. J Cell Sci. doi.org/10.1242/jcs.228833
Gönczy, P., Rose, LS. 2005. Asymmetric cell division and axis formation in the embryo. WormBook. doi.org/10.1895/wormbook.1.30.1
Gurdon, J.B. 1960. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J Embryol Exp Morphol. Dec;8:505-26.
Gurdon, JB., Wilmut, I. 2011. Nuclear transfer to eggs and oocytes. Cold Spring Harb Perspect Biol. doi.org/10.1101/cshperspect.a002659
Ham, Arthur W. (Arthur Worth), David H. Cormack. 1987. Ham’s Histology. 9th ed. Philadelphia: Lippincott. Print.
Han et al. 2020. Construction of a human cell landscape at single-cell level. Nature. doi.org/10.1038/s41586-020-2157-4
Hansis, C. 2006. Totipotency, cell differentiation and reprogramming in humans. Reprod Biomed Online. doi.org/10.1016/s1472-6483(10)60644-x
Hartung, M., Stahl, A. 1977. Preleptotene chromosome condensation in mouse oogenesis. Cytogenet Cell Genet. doi.org/10.1159/000130777
Hayflick, L. 1997. Mortality and immortality at the cellular level. A review. Biochemistry (Mosc). Nov;62(11):1180-90.
Hayflick L. The greatest risk factor for the leading cause of death is ignored. Biogerontology. 2021. doi: 10.1007/s10522-020-09901-y
Hinchcliffe et al. 2001. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. doi.org/10.1126/science.1056866
Hwang et al. 2013. Successful cloning of coyotes through interspecies somatic cell nuclear transfer using domestic dog oocytes. Reprod Fertil Dev. doi.org/10.1071/rd12256
Ishiuchi, T., Torres-Padilla, ME. 2013. Towards an understanding of the regulatory mechanisms of totipotency. Curr Opin Genet Dev. doi.org/10.1016/j.gde.2013.06.006
Jaba, T. (2022). Dasatinib and quercetin: short-term simultaneous administration yields senolytic effect in humans. Issues and Developments in Medicine and Medical Research Vol. 2, 22-31. doi: https://doi.org/10.9734/bpi/idmmr/v2/15155D
Khan, YS., Farhana, A. Histology, Cell. 2022. May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32119269.
Lanza et al. 2000. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning. doi.org/10.1089/152045500436104
Lavasani et al. 2012. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. doi: 10.1038/ncomms1611
Le Guennec et al. 2021. Overview of the centriole architecture. Curr Opin Struct Biol. 2021 Feb;66:58-65. doi: 10.1016/j.sbi.2020.09.015
Lezhava, T., Monaselidze, J., Jokhadze, T., Kakauridze, N., Khodeli, N., Rogava, M., Tkemaladze J., ... & Gaiozishvili, M. (2011). Gerontology research in Georgia. Biogerontology, 12, 87-91. doi: 10.1007/s10522-010-9283-6. Epub 2010 May 18. PMID: 20480236; PMCID: PMC3063552
Loi et al. 2001. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol. doi.org/10.1038/nbt1001-962
López-Otín et al. 2013. The Hallmarks of Aging. doi.org/10.1016/J.CELL.2013.05.039
Maemura et al. 2021. Totipotency of mouse zygotes extends to single blastomeres of embryos at the four-cell stage. Sci Rep. dx.doi.org/10.1038%2Fs41598-021-90653-1
Manandhar, G., Schatten, H., Sutovsky, P. 2005. Centrosome reduction during gametogenesis and its significance. Biol Reprod. doi.org/10.1095/biolreprod.104.031245
Maniotis, A., Schliwa, M. 1991. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. doi: 10.1016/0092-8674(91)90524-3
Maro et al. 1991. Cell polarity and microtubule organisation during mouse early embryogenesis. Dev Suppl.;1:17-25.
Marshall, WF., Rosenbaum, JL. 2000. Are there nucleic acids in the centrosome? Curr Top Dev Biol. doi.org/10.1016/s0070-2153(99)49009-x
Matsaberidze, M., Prangishvili, A., Gasitashvili, Z., Chichinadze, K., & Tkemaladze, J. (2017). To topology of anti-terrorist and anti-criminal technology for educational programs. International Journal of Terrorism & Political Hot Spots, 12.
Molofsky et al. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. doi: 10.1038/nature05091
Moore, JA. 1960. Serial back-transfers of nuclei in experiments involving two species of frogs. Dev Biol. doi.org/10.1016/0012-1606(60)90053-1
Murugan et al. 2022. Acute multidrug delivery via a wearable bioreactor facilitates long-term limb regeneration and functional recovery in adult Xenopus laevis. Sci Adv. doi.org/10.1126/sciadv.abj2164
Nabais et al. 2021. Plk4 triggers autonomous de novo centriole biogenesis and maturation. J Cell Biol. doi: 10.1083/jcb.202008090
Narbonne, P., Miyamoto, K., Gurdon, JB. 2012. Reprogramming and development in nuclear transfer embryos and in interspecific systems. Curr Opin Genet Dev. doi.org/10.1016/j.gde.2012.09.002
Nigg, EA., Holland, AJ. 2018. Once and only once: mechanisms of centriole duplication and their deregulation in disease. Nat Rev Mol Cell Biol. doi.org/10.1038/nrm.2017.127
Pechersky et al. 2016. Immune System and Regeneration. J Stem Cells. 2016;11(2):69-87. PMID: 28296866.
Prangishvili, A., Gasitashvili, Z., Matsaberidze, M., Chkhartishvili, L., Chichinadze, K., Tkemaladze, J., ... & Azmaiparashvili, Z. (2019). System components of health and innovation for the organization of nano-biomedic ecosystem technological platform. Current Politics and Economics of Russia, Eastern and Central Europe, 34(2/3), 299-305.
Roy, S., Kundu, TK. 2014. Gene regulatory networks and epigenetic modifications in cell differentiation. IUBMB Life. doi: 10.1002/iub.1249
Schwarz et al. 2018. Revisiting Centrioles in Nematodes-Historic Findings and Current Topics. Cells.doi.org/10.3390/cells7080101
Seirin-Lee, S. 2020. Asymmetric cell division from a cell to cells: Shape, length, and location of polarity domain. Dev Growth Differ. dx.doi.org/10.1111%2Fdgd.12652
Shankar et al. 2021. From Snapshots to Development: Identifying the Gaps in the Development of Stem Cell-based Embryo Models along the Embryonic Timeline. Adv Sci (Weinh). doi.org/10.1002/advs.202004250
Shaw et al. 2010. Aging of the innate immune system. Curr Opin Immunol. doi:10.1016/j.coi.2010.05.003
Silva et al. 2011. Why are monozygotic twins different? J Perinat Med. doi.org/10.1515/jpm.2010.140
Simerly et al. 2016. Post-Testicular Sperm Maturation: Centriole Pairs, Found in Upper Epididymis, are Destroyed Prior to Sperm's Release at Ejaculation. Sci Rep. doi.org/10.1038/srep31816
Simerly et al. 2018. Separation and Loss of Centrioles From Primordidal Germ Cells To Mature Oocytes In The Mouse. Sci Rep. doi.org/10.1038/s41598-018-31222-x
Sobhani et al. 2017. Multipotent Stem Cell and Current Application. Acta Med Iran. Jan;55(1):6-23.
Spalding et al. 2005. Retrospective birth dating of cells in humans. Cell. doi:10.1016/j.cell.2005.04.028
Tkemaladze, J., & Chichinadze, K. (2005). Potential role of centrioles in determining the morphogenetic status of animal somatic cells. Cell biology international, 29(5), 370-374. doi: 10.1016/j.cellbi.2005.03.003. PMID: 15886028.
Tkemaladze, J. V., & Chichinadze, K. N. (2005). Centriolar mechanisms of differentiation and replicative aging of higher animal cells. Biochemistry (Moscow), 70, 1288-1303. doi: 10.1007/s10541-005-0261-6. PMID: 16336191
Tkemaladze, J., & Chichinadze, K. (2010). Centriole, differentiation, and senescence. Rejuvenation research, 13(2-3), 339-342. doi: 10.1089/rej.2009.0904. PMID: 20426623
Tkemaladze, J., Tavartkiladze, A., & Chichinadze, K. (2012). Programming and Implementation of Age-Related Changes. In Senescence. IntechOpen. DOI: 10.5772/33420
Tkemaladze, J., & Apkhazava, D. (2019). Dasatinib and quercetin: short-term simultaneous administration improves physical capacity in human. J Biomedical Sci, 8(3), 3.
Tkemaladze, J. (2022). Long-Term Differences between Regenerations of Head and Tail Fragments in Schmidtea Mediterranea Ciw4. Available at SSRN 4257823.
Tkemaladze, J. (2023). Reduction, proliferation, and differentiation defects of stem cells over time: a consequence of selective accumulation of old centrioles in the stem cells?. Molecular Biology Reports, 50(3), 2751-2761.doi: 10.1007/s11033-022-08203-5. Epub 2022 Dec 30. PMID: 36583780
Tkemaladze, J. (2023). The centriolar hypothesis of differentiation and replicative senescence. Junior Researchers, 1(1). doi: https://doi.org/10.52340/2023.01.01.15
Tkemaladze, J. (2023). Structure and possible functions of centriolar RNA with reference to the centriolar hypothesis of differentiation and replicative senescence. Junior Researchers, 1(1), 156–170. https://doi.org/10.52340/2023.01.01.17
Tkemaladze, J. (2023). Cross-Senolytic Effects of Dasatinib and Quercetin in Humans. Georgian Scientists. 5 (3):138-52. https://doi.org/10.52340/2023.05.03.15
Venkei, ZG., Yamashita, YM. 2018. Emerging mechanisms of asymmetric stem cell division. J Cell Biol. doi.org/10.1083/jcb.201807037
Wadsworth, P., Khodjakov, A. 2004. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. doi.org/10.1016/j.tcb.2004.07.004
Weismann, A. 1890. Prof. Weismann's Theory of Heredity. Nature 41,317–323. doi:10.1038/041317g0
Wen, W. Phase Separation in Asymmetric Cell Division. 2020. Biochemistry. doi.org/10.1021/acs.biochem.9b00813
Woodruff, JB., Wueseke, O., Hyman, AA. 2014. Pericentriolar material structure and dynamics. Philos Trans R Soc Lond B Biol Sci. doi.org/10.1098/rstb.2013.0459
Zhou, LQ., Dean J. 2015. Reprogramming the genome to totipotency in mouse embryos. Trends Cell Biol. doi.org/10.1016/j.tcb.2014.09.006
Прангишвили, А. И., Гаситашвили, З. А., Мацаберидзе, М. И., Чичинадзе, К. Н., Ткемаладзе, Д. В., & Азмайпарашвили, З. А. (2017). К топологии антитеррористических и антикриминальных технологии для образовательных программ. In Управление развитием крупномасштабных систем MLSD'2017 (pp. 284-287).
Прангишвили, А. И., Гаситашвили, З. А., Мацаберидзе, М. И., Чхартишвили, Л. С., Чичинадзе, К. Н., Ткемаладзе, Д. В., ... & Азмайпарашвили, З. А. (2017). Системные составляющие здравоохранения и инноваций для организации европейской нано-биомедицинской екосистемной технологической платформы. In Управление развитием крупномасштабных систем MLSD'2017 (pp. 365-368).
Ткемаладзе Д. , Цомаиа Г ., Жоржолиани И. (2001). Создание искусственных самоадаптирующихся систем на основе Теории Прогноза. Искусственный интеллект. УДК 004.89. Искусственный интеллект. УДК 004.89. https://www.ipai.net.ua/uk/arch-2001-3#
Ткемаладзе, Д. В., & Чичинадзе, К. Н. (2005). Центриолярные механизмы дифференцировки и репликативного старения клеток высших животных. Биохимия, 70(11), 1566-1584.
Чичинадзе, К., Ткемаладзе, Д., & Лазарашвили, А. (2012). Новый класс рнк и центросомная гипотеза старения клеток. Успехи геронтологии, 25(1), 23-28.
Чичинадзе, К. Н., & Ткемаладзе, Д. В. (2008). Центросомная гипотеза клеточного старения и дифференциации. Успехи геронтологии, 21(3), 367-371
Copyright (c) 2023 GEORGIAN SCIENTISTS

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.