INTERACTIONS OF BIOFLAVONOID ROBININ WITH EFFLUX TRANSPORTERS: P-GLYCOPROTEIN AND BREAST CANCER RESISTANCE PROTEIN
Downloads
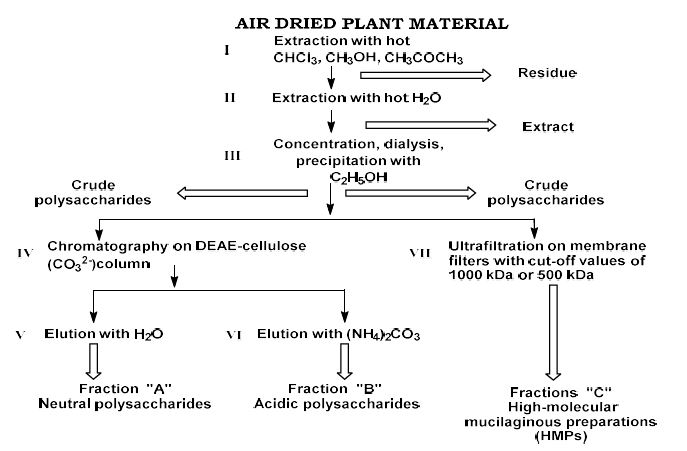
Present study investigated the possible role of P-glycoprotein in the intracellular exposure of active substance (robinin) of natural medicine Flaroninum™ and effect of robinin on the cellular accumulation of mitoxantrone in either BCRP-overexpressing and BCRP-negative cells. Robinin - kaempferol-3-O-b-D-robinoside-7-O-α-L-rhamnopyranoside, has been obtained from over ground parts of Astragalus falcatus Lam., growing in Georgia. The compound possesses hypoazotemic and diuretic activities and is proposed for the complex therapy of kidney diseases. P-gp-overexpressing and P-gp-negative human breast cancer cells (MCF7) were used to examine the absorption character of kaempferol and robinin with or without of verapamil (P-gp inhibitor). Flavonoids concentrations were determined by LC/MS/MS. Robinin transport was P-gp-dependent and verapamil significantly increased the extent of uptake of robinin in cells, which is attributed in part to P-gp-inhibition by verapamil. When comparing the intracellular accumulation of aglycone with kaempferol-3-O-b-D-robinoside-7-O-α-L-rhamnopyranoside, we found that kaempferol uptake was greater than robinin. Since some glycosylated forms of flavonoids have been demonstrated to significantly suppress breast cancer resistance protein (BCRP/ABCG2), we hypothesized that kaempferol-3-O-b-D-robinoside-7-O-α-L-rhamnopyranoside may act as a BCRP-inhibitor. Mitoxantrone accumulation studies were performed in BCRP-overexpressing and BCRP-negative MCF7 cells using flow cytometric analysis. Robinin (5 and 50 mM) had no significant impacts on the BCRP-mediated mitoxantrone transport; on the contrary, the aglycone kaempferol produced increased accumulation of mitoxantrone. From these results, we suggest that the active component of natural hypoazotemic medicine Flaroninum™, robinin, may be a substrate of P-gp and its efflux outside of the cells mediated P-gp may contribute to its decreased intracellular concentration.
Downloads
Mateus, A.; Treyer, A.; Wegler, C.; Karlgren, M.; Matsson, P.; Artursson, P. Intracellular drug bioavailability: a new predictor of system dependent drug disposition. Sci Rep. 2017, 7:43047. doi:10.1038/srep43047
Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M. L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules. 2017, 22(4):600. doi:10.3390/molecules22040600
Blanco, V.E.; Hernandorena, C.V.; Scibona, P.; Belloso, W.; Musso, C.G. Acute Kidney Injury Pharmacokinetic Changes and Its Impact on Drug Prescription. Healthcare. 2019, 7(1):10. doi:10.3390/healthcare7010010
Drozdzik, M.; Drozdzik, M.; Oswald, S. Membrane Carriers and Transporters in Kidney Physiology and Disease. Biomedicines 2021, 9: 426. doi:10.3390/biomedicines9040426
Droździk, M.; Oswald, S.; Droździk, A. Impact of kidney dysfunction on hepatic and intestinal drug transporters. Biomed Pharmacother. 2021, 143:112125. doi:10.1016/j.biopha.2021.112125.
Atilano-Roque, A.; Roda, G.; Fogueri, U.; Kiser, J.J.; Joy, M.S. Effect of Disease Pathologies on Transporter Expression and Function. J Clin Pharmacol. 2016, 56(7):S205-21. doi: 10.1002/jcph.768.
Torres, A.M.; Dnyanmote, A.V.; Granados, J.C.; Nigam, S.K. Renal and non-renal response of ABC and SLC transporters in chronic kidney disease. Expert Opin Drug Metab Toxicol. 2021, 17(5):515-542. doi: 10.1080/17425255.2021.1899159.
Saad, R.; Hallit, S.; Chahine, B. Evaluation of renal drug dosing adjustment in chronic kidney disease patients at two university hospitals in Lebanon. Pharm Pract. 2019, 17(1):1304. doi:10.18549/PharmPract.2019.1.1304.
Vesga, J.I.; Cepeda, E.; Pardo, C.E.; Paez, S.; Sanchez, R.; Sanabria, R.M. Chronic Kidney Disease Progression and Transition Probabilities in a Large Preventive Cohort in Colombia. Int J Nephrol. 2021, 2021: 8866446. doi:10.1155/2021/8866446.
Thurlow, J.S; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am J Nephrol. 2021, 52(2):98-107. doi: 10.1159/000514550.
Chen, D.Q.; Hu, H.H.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine. 2018, 50: 50-60. doi:10.1016/j.phymed.2018.09.182
Shebeko, S.K.; Chernykh, V.V.; Zupanets, K.O. Nephroprotective Effect of the Herbal Composition BNO 2103 in Rats with Renal Failure. Sci. Pharm. 2020, 88:47. doi:10.3390/scipharm88040047
Mondal, S.; Rahaman, S.T. Flavonoids: A vital resource in healthcare and medicine. Pharm Pharmacol Int J. 2020, 8(2): 91‒104. doi: 10.15406/ppij.2020.08.00285
Gomes, A.; Fernandes, E; Lima, J.L.; Mira, L.; Corvo, M.L. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr Med Chem. 2008, 15(16):1586-605. doi: 10.2174/092986708784911579
Tsuji, P.A; Stephenson, K.K; Wade, K.L; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr Cancer. 2013, 65(7):1014-25. doi: 10.1080/01635581.2013.809127
Tang, D.; Zhu, J. X.; Nie, H.; He, B.; Xu, Y. H.; Zhu, Q. Simple and efficient approach for enrichment of major isoflavonoids from Astragalus membranaceus with macroporous resins and their nephroprotective activities. Ind Crops Prod. 2018, 125: 276–283. doi:10.1016/j.indcrop.2018.08.023
Jiang, W.; Hu, M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012, 2(21):7948-7963. doi:10.1039/C2RA01369J
Dietrich, C. G.; Geier, A.; Oude Elferink, R. P. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003, 52(12):1788–1795. doi:10.1136/gut.52.12.1788
Zhang, S.; Yang, X.; Morris, M. E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004, 65(5): 1208–1216. doi:10.1124/mol.65.5.1208
Nguyen, T.T.; Duong, V.A; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics. 2021; 13(7):1103. doi:10.3390/pharmaceutics13071103
Amin, M.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target. Insights ; 2013, 7: 27–34.
Zhang, H.W; Lin, Z.X.; Xu, C.; Leung, C.; Chan, L.S. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014, (10):CD008369. doi:10.1002/14651858.CD008369.
Okuda, M.; Horikoshi, S.; Matsumoto, M.; Tanimoto, M.; Yasui, H.; Tomino, Y. Beneficial effect of Astragalus membranaceus on estimated glomerular filtration rate in patients with progressive chronic kidney disease. Hong Kong J Nephrol. 2012, 14(1): 17–23. doi:10.1016/j.hkjn.2012.01.001
Bratkov, V. M.; Shkondrov, A. M.; Zdraveva, P. K.; Krasteva, I. N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharmacogn Rev. 2016, 10(19):11–32. doi:10.4103/0973-7847.176550
Lysiuk, R.; Darmohray, R. Pharmacology and Ethnomedicine of the Genus Astragalus. Int. j. pharmacol. phytochem. ethnomed. 2016, 3:46–53. doi:10.18052/www.scipress.com/IJPPE.3.46
Alaniya, M.D.; Sutiashvili, M.G.; Kavtaradze, N.Sh.;Skhirtladze, A.V. Chemical constituents of Astragalus falcatus. Chem Nat Compd. 2017, 53:1202–1203. doi:10.1007/s10600-017-2240-8
Kemertelidze, E.; Alania, M.; Sagareishvili, T.; Shalashvili, K.; Kavtaradze, N. Medicinal preparations on the basis of vegetable phenolic compounds. Planta Med. 2009, 75, PD62.
Gigoshvili, T.; Alaniya, M. Flavonoids and cycloartans in the production waste of “Flaronin” preparation. Proc. Georgian Acad. Sci., Chemical Series. 1996, 22(1-4):176–178 (Georgian) http://dspace.nplg.gov.ge/bitstream/1234/188730/1/Macne_1996_N1-4.pdf
Kemertelidze, E.P.; Syrov, V.N.; Alaniya, M.D.; Kavtaradze, N.Sh.; Khushbaktova, Z.A. Chemical composition and pharmacological activity of the leaves Pueraria hirsuta L. growing in Georgia. Pharm Chem J. 2008, 42: 340-34. doi: 10.1007/s11094-008-0131-9
Lau, C. S.; Carrier, D. J.; Beitle, R. R.; Howard, L. R.; Lay, J. O.; Liyanage, R.; Clausen, E. C. A glycoside flavonoid in Kudzu (Pueraria lobata): identification, quantification, and determination of antioxidant activity. Appl Biochem Biotechnol. 2005, 121-124: 783–794. doi:10.1385/abab:123:1-3:0783
Yahara, S.; Kohjyouma, M.; Kohoda, H. Flavonoid glycosides and saponins from Astragalus shikokianus. Phytochemistry. 2000, 53(4):469–471. doi:10.1016/s0031-9422(99)00512-9
Akhmedzhanova, V. Robinin and kaempfereol from Vinca erecta. Chem Nat. Compd. 1987, 22(5): 601-602. doi.org/10.1007/BF00599275
Bokkenheuser, V. D.; Shackleton, C. H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248(3): 953–956. doi:10.1042/bj2480953
An, G.; Gallegos, J.; Morris, M. E. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos 2011, 39(3): 426–432. doi:10.1124/dmd.110.035212
Fang, Y.; Xia, M.; Liang, F.; Cao, W.; Pan, S.; Xu, X. Establishment and use of human mouth epidermal carcinoma (KB) cells overexpressing P-glycoprotein to characterize structure requirements for flavonoids transported by the efflux transporter. J Agric Food Chem. 2019, 67(8):2350-2360. doi: 10.1021/acs.jafc.9b00039.
Zhang, S.; Morris, M. E. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J. Pharmacol. Exp. Ther. 2002, 304 (3): 1258−1267.
Singh, S.; Prasad, N. R.; Chufan, E. E.; Patel, B. A.; Wang, Y. J.; Chen, Z. S.; Ambudkar, S. V.; Talele, T. T. Design and synthesis of human ABCB1 (P-glycoprotein) inhibitors by peptide coupling of diverse chemical scaffolds on carboxyl and amino termini of (S)- valine-derived thiazole amino acid. J. Med. Chem. 2014, 57 (10):4058−4072.
Kadioglu, O.; Saeed, M. E.; Valoti, M.; Frosini, M.; Sgaragli, G.; Efferth, T. Interactions of human P-glycoprotein transport substrates and inhibitors at the drug binding domain: Functional and molecular docking analyses. Biochem.Pharmacol. 2016, 104: 42−51.
Yuan, Z. W.; Li, Y. Z.; Liu, Z. Q.; Feng, S. L.; Zhou, H.; Liu, C. X.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol.Res. 2018, 128:153−166.
Tsiklauri, L.; Švík, K.; Chrastina, M.; Poništ, S.; Dráfi, F.; Slovák, L.; Alania, M.; Kemertelidze, E.; Bauerova, K. Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients 2021, 13:1268. doi:10.3390/nu13041268
Minderman, H.; Suvannasankha, A.; O'Loughlin, K. L.; Scheffer, G. L.; Scheper, R. J.; Robey, R. W.; Baer, M. R. Flow cytometric analysis of breast cancer resistance protein expression and function. Cytometry. 2002, 48(2): 59–65. doi:10.1002/cyto.10111
Tsiklauri, L.; An, G.; Ruszaj, D. M.; Alaniya, M.; Kemertelidze, E.; Morris, M. E. Simultaneous determination of the flavonoids robinin and kaempferol in human breast cancer cells by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2011, 55(1): 109–113. doi:10.1016/j.jpba.2010.12.021
Fairchild, C. R.; Moscow, J. A.; O'Brien, E. E.; Cowan, K. H. Multidrug resistance in cells transfected with human genes encoding a variant P-glycoprotein and glutathione S-transferase-pi. Mol Pharmacol. 1990, 37(6):801–809.
Jiang, J.; Wang, X.; Cheng, K.; Zhao, W.; Hua, Y.; Xu, C.; Yang, Z. Psoralen reverses the P-glycoprotein-mediated multidrug resistance in human breast cancer MCF-7/ADR cells. Mol Med Rep. 2016, 13:4745-4750. doi:10.3892/mmr.2016.5098
Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-glycoprotein inhibitory potential using a rhodamine 123 accumulation assay. Pharmaceutics. 2016, 8:12.
Walgren, R. A.; Walle, U. K.; Walle, T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem Pharmacol. 1998, 55(10): 1721–1727. doi:10.1016/s0006-2952(98)00048-3
Zhang, H.; Hassan, Y. I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular Mechanisms Underlying the Absorption of Aglycone and Glycosidic Flavonoids in a Caco-2 BBe1 Cell Model. ACS Omega. 2020, 5(19):10782–10793. doi:10.1021/acsomega.0c00379
Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit Rev Food Sci Nutr. 2017, 57(9): 1874–1905. doi:10.1080/10408398.2015.1032400
Katayama, K., Masuyama, K.; Yoshioka, S.; Hasegawa, H.; Mitsuhashi, J.; Sugimoto, Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: transporter specificity and structure-activity relationship. Cancer Chemother Pharmacol. 2007, 60(6):789–797. doi:10.1007/s00280-007-0426-7
Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res 2004, 64(12): 4346–4352. doi:10.1158/0008-5472.CAN-04-0078
Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E. M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R. D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother Res. 2017, 31(10):1529–1538. doi:10.1002/ptr.5878
Fleisher, B.; Unum, J.; Shao, J.; An, G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci. 2015, 104(1):266–275. doi:10.1002/jps.24289
Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; Li, Y. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicol in Vitro. 2019, 61:104642. doi:10.1016/j.tiv.2019.104642
Sjöstedt, N.; Holvikari, K.; Tammela, P.; Kidron, H. Inhibition of Breast Cancer Resistance Protein and Multidrug Resistance Associated Protein 2 by Natural Compounds and Their Derivatives. Mol Pharm. 2017, 14 (1):135–146. doi:10.1021/acs.molpharmaceut.6b00754
Zhang, S.; Yang, X.; Morris, M. E. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004, 21(7): 1263–1273. doi:10.1023/b:pham.0000033015.84146.4c
Copyright (c) 2023 GEORGIAN SCIENTISTS

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.