Physicochemical characteristics of fresh and lyophilized Georgian royal jelly and Formulation bentonite-based cream
Downloads
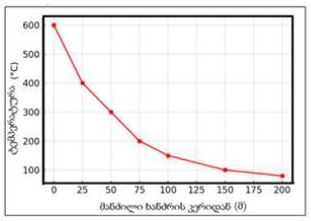
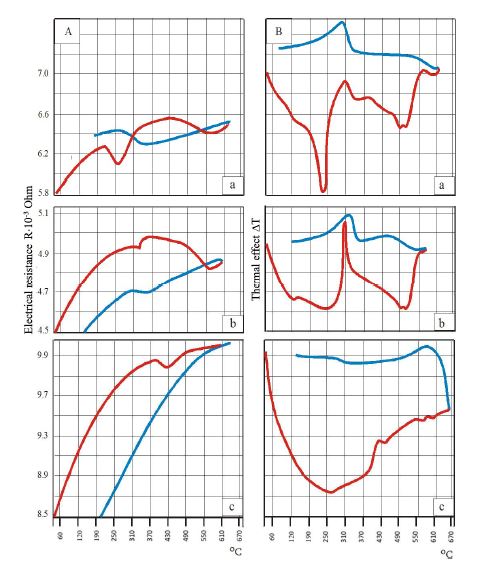
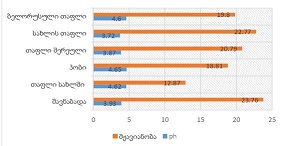
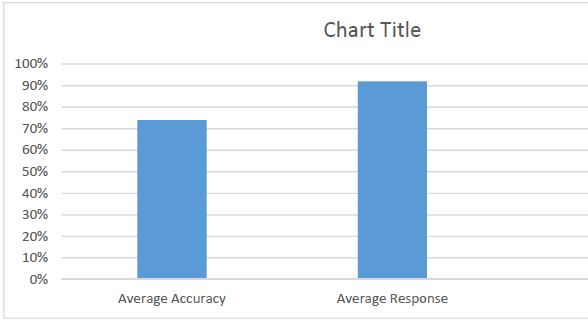
Royal Jelly (RJ) is one of the most valued and studied bee products used in medicine and cosmetics due to its diverse pharmacological properties. Chemical composition and biological activity of RJ depends on many factors, including constitution and geographical distribution of plants, the harvest time and bee species. The objective of the present study was physico-chemical characterization and comparison of fresh and lyophilized RJ; and development dermatological formulation by incorporating RJ in Tikha Ascane base. The evaluations of RJ and RJ loaded formulations were performed by oven-dry, titrimetric, potentiometric, FTIR methods; 10-hydroxy-2-decenoic acid (10-HDA) was determined by high-performance liquid chromatography. In addition, the physical stability of the developed formulations was investigated by examining viscosity, spreadability, thermal and colloidal stabilities, pH, homogeneity and appearance. Lyophilized RJ displayed up to similar physico-chemical properties (solubility, pH, acidic number) as fresh RJ. The chromatographic analysis showed alike fingerprints and the existence of 10-HAD in tested samples. No spectral differences (in the range of 3600–2800 cm-1 and 1750–950 cm-1) were observed in FTIR measurements after lyophilization. The study shows that prepared formulations were found to be stable. The tested samples had appealing appearance with smooth texture and no signs of separation. Viscosity, homogeneity, spreadability, pH and release profile of RJ from the formulations were acceptable.
Downloads
Ahmad S, Campos MG, Fratini F, Altaye SZ, Li J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int J Mol Sci. 2020 ; 8;21(2):382.
Khazaei M, Ansarian A, Ghanbari E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J Diet Suppl. 2018; 15(5):757-775.
Kausar SH, More VR. Royal Jelly: Organoleptic characteristics and physicochemical properties. Lipids. 2019; 6(2):20-4.
Kirkitadze G, Japoshvili G. Renewed checklist of bees (Hymenoptera, Andrenidae) from Georgia. Annals of agrarian science. 2015; 13: 20-32.
Maghsoudlou A, Mahoonak AS, Mohebodini H, Toldra F. Royal Jelly: Chemistry, Storage and Bioactivities, Journal of Apicultural Science. 2019; 63 (1):17-40.
Lazarevska S, Makreski P. Insights into the infrared and Raman spectra of fresh and lyophilized royal jelly and protein degradation IR spectroscopy study during heating, Macedonian Journal of Chemistry and Chemical Engineering. 2015; 34(1): 87–93.
Babahoum, N., Ould Hamou, M. Characterization and purification of Algerian natural bentonite for pharmaceutical and cosmetic applications. BMC Chemistry 15, 50 (2021). https://doi.org/10.1186/s13065-021-00776-9
Cervini-Silva J, Antonio-Nieto-Camacho, Kaufhold S, Ufer K, de Jesús ER. (The anti-inflammatory activity of bentonites. Applied Clay Science. 2015; 118:56–60. https://doi.org/10.1016/j.clay.2015.08.039
Tsagareishvili GV. Some results of research and application of bentonites of Georgia in pharmacy and medicine" - Tbilisi. "Metsniereba". 1974. 130 (in Russian).
Aladishvili V.A. The use of bentonite of the Askane deposit in medicine. Tbilisi. 1969. 24-27 (in Russian) .
Tsiklauri L, Dadeshidze I, Tsagareishvili G. Study of the Stability and Specific Activity of the Emulsion Containing Sea-Buckthorn (Hippophae rhamnoides L.) Oil and Ticha - Ascanae. Bulletin of the Georgian Academy of Sciences. 1998; 157(2):251-253.
Tsiklauri L, Tsagareishvili G. Gastroenterological and dermatological soft drug formulations containing Georgian bentonite clay preparation – Thicha-ascanae. Tbilisi. “Universal”. 2011, 89, (ISBN 978-9941-17-458-2) (in Georgian).
Kanelis D, Tananaki C, Liolios V, et al. A suggestion for royal jelly specifications. Arh Hig Rada Toksikol. 2015; 66(4):275-284. doi:10.1515/aiht-2015-66-2651
Balkanska R. Determination of Trans-10-Hydroxy-2-Decenoic Acid in Royal Jelly by High Performance Liquid Chromatography after Different Bee Feeding. Int. J. Curr. Microbiol. App.Sci. 2018; 7(4): 3738-3743
Bocho-Janiszewska A, Sikora A, Rajewski J, Łobodzin P. Application of royall jelly in moisturizing creams. Pol. J. Cosmetol. 2013; 16: 314–320
Chen M X, Alexander K., Baki G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. Journal of Pharmaceutics, 2016; 1–10. doi:10.1155/2016/5754349
Knorst M.T., Desenvolvimento tecnológico de forma farmacêutica plástica contendo extrato concentrado de Achyrocline satureioides. (Lam). DC. Compositae. (Marcela), M. Sc. thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, 1991, p. 257
Khan MFA, Ur.Rehman A, Howari H, Alhodaib A, Ullah F, Mustafa Zu, Elaissari A, Ahmed N. Hydrogel Containing Solid Lipid Nanoparticles Loaded with Argan Oil and Simvastatin: Preparation, In Vitro and Ex Vivo Assessment. Gels. 2022; 8(5):277. https://doi.org/10.3390/gels8050277
Boselli E, Caboni MF, Sabatini AG, Marcazzan GL, Lercker G. Determination and changes of free amino acids in royal jelly during storage, Apidologie. 2003; 34: 129 – 137
Ali, S.M. and G. Yosipovitch, Skin pH: from basic science to basic skin care. Acta dermato-venereologica, 2013; 93(3): 261-269
Pattanayek S, Puranik S. Formulation and Evaluation of Ketoprofen Loaded Nanoparticulate Gel for Topical Delivery. IJPPR. 2018; 11(3):1–11.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.