THE SCIENTIFIC DISCUSSION OF SOME ISSUES OF FEATURES AND CHALLENGES OF USING OF CAR-T CELLS IN IMMUNOTHERAPY
Downloads
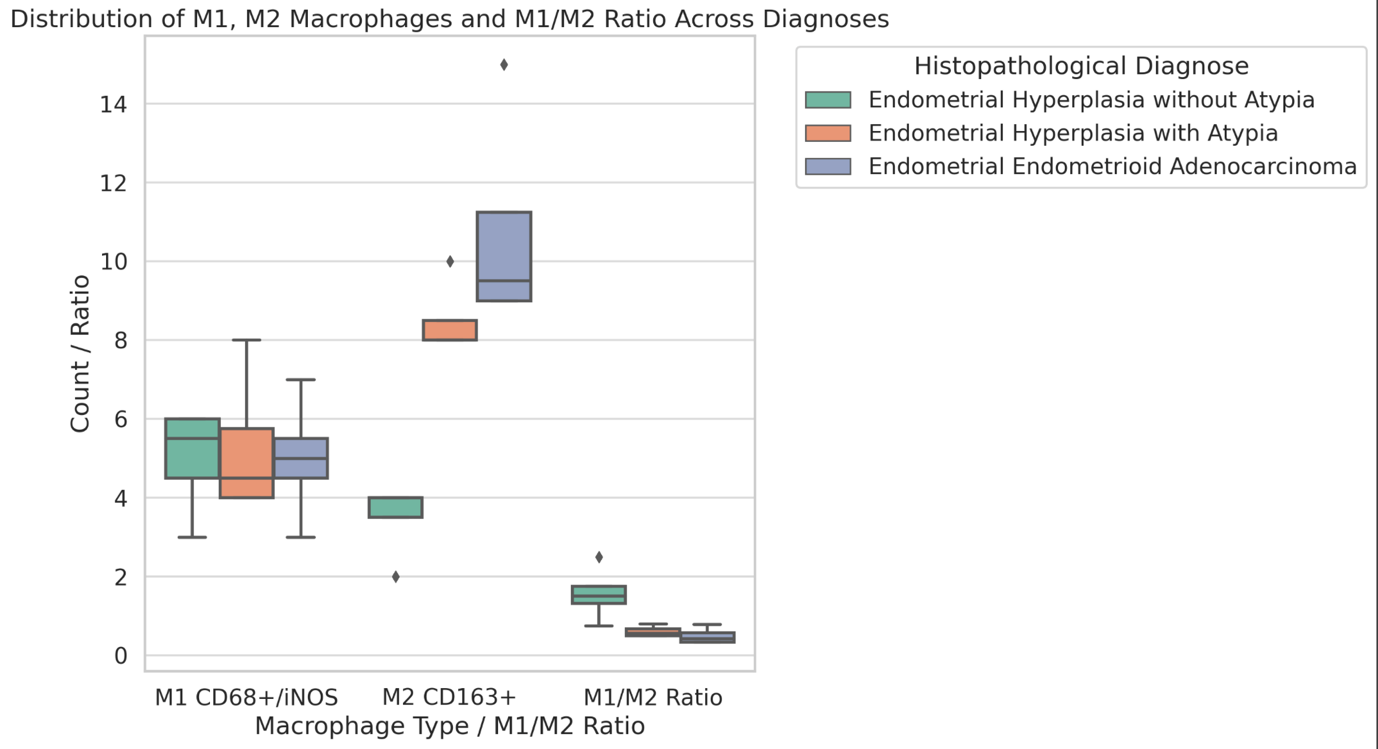
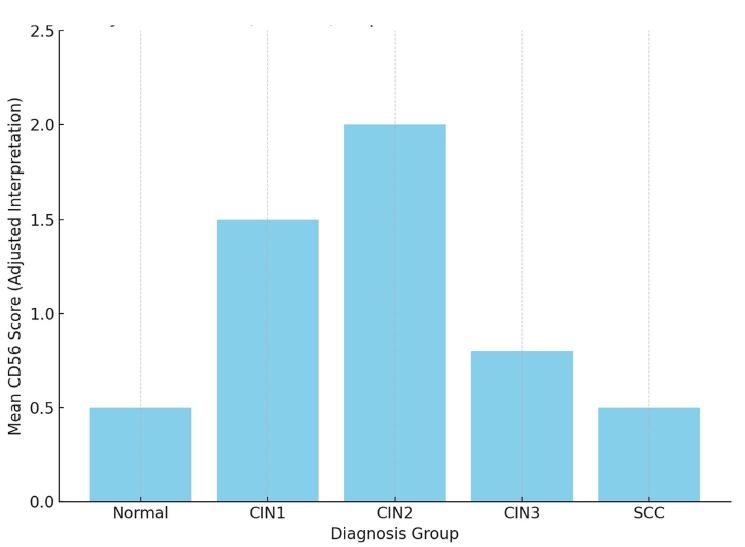
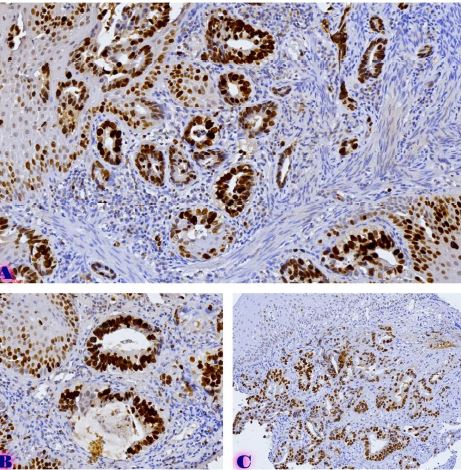
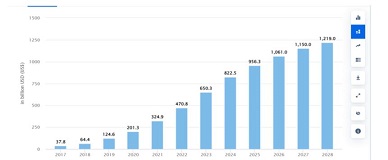
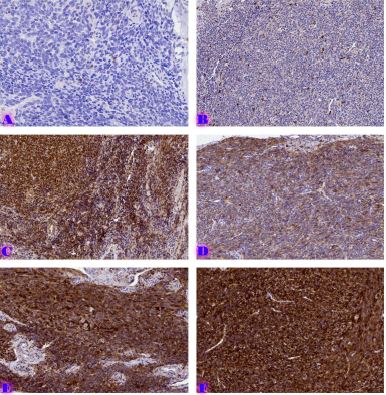
Aim of the research was to study and analyze some issues of features of using of car-t cells in immunotherapy. The characterization of CAR-T cell products involves evaluating their phenotype, genotype, and functional attributes using techniques such as flow cytometry, PCR-based assays, and cytotoxicity assays. Long-term stability studies assess product viability, potency, and cytokine secretion profiles across different storage conditions to determine shelf-life and facilitate product logistics. Biomarkers serve as crucial tools in the field of cancer immunotherapy, aiding in patient selection and treatment optimization. In the context of chimeric antigen receptor (CAR) T cell therapy, biomarkers play a significant role in predicting treatment response, identifying potential toxicities, and guiding personalized treatment approaches. Predictive biomarkers in CAR-T cell therapy commonly center on the profiles of tumor antigen expression. The selection of target antigens plays a crucial role in treatment outcomes, with higher and more consistent expression levels correlating with better response rates. The tumor microenvironment (TME) significantly influences CAR-T cell activity. Biomarkers reflecting TME features, such as immune cell infiltration, cytokine profiles, and expression of inhibitory molecules, offer insights into the immunosuppressive nature of the TME and its effects on CAR-T cell effectiveness. Patients with an inflamed TME, marked by abundant effector T cells and low expression of inhibitory molecules like PD-L1, tend to respond better to CAR-T cell therapy. CAR-T cells demonstrate bystander killing effects, where nearby tumor cells without the target antigen are eradicated via a phenomenon called antigen spreading. This process is triggered by the release of cytokines and the presentation of tumor antigens by antigen-presenting cells (APCs), resulting in the activation of the body's own immune effector cells against the tumor cells. CAR-T cells exhibit strong antitumor capabilities, they can face resistance mechanisms within the tumor microenvironment. The immunosuppressive cell populations like regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), as well as inhibitory cytokines such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10). The resistance mechanisms, strategies involve employing combination therapies with immune checkpoint inhibitors, cytokine modulators, and targeted therapies aimed at disrupting immunosuppressive pathways. CAR-T cell therapy has revolutionized cancer treatment by harnessing the power of the immune system to target and eliminate tumor cells.
Downloads
Gross, G., Waks, T., & Eshhar, Z. (1989). Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences, 86(24), 10024–10028.
Levine, B. L., Miskin, J., Wonnacott, K., Keir, C., & Global, L. (2016). Global manufacturing of CAR T cell therapy. Molecular Therapy – Methods & Clinical Development, 4, 92–101.
Lai, Y., Weng, J., Wei, X., Qin, L., Lai, P., Zhao. (2018). Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T cells. Leukemia, 32(3), 801–808.
Guedan, S., Calderon, H., Posey, M. V., & June, C. H. (2019). Engineering and Design of Chimeric Antigen Receptors. Molecular Therapy – Methods & Clinical Development, 12, 145–156.
Ren, J., Zhang, X., Liu, X., Fang, C., Jiang, S., June, C. H., & Zhao, Y. (2017). A Versatile System for Rapid Multiplex Genome-edited CAR T Cell Generation. Oncotarget, 8(10), 17002–17011.
June, C. H., & Sadelain, M. (2018). Chimeric Antigen Receptor Therapy. New England Journal of Medicine, 379(1), 64–73.
Zah, E., Lin, M. Y., Silva-Benedict, A., Jensen, M. C., & Chen, Y. Y. (2016). T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunology Research, 4(6), 498–508.
Fesnak, A. D., June, C. H., & Levine, B. L. (2016). Engineered T cells: The promise and challenges of cancer immunotherapy. Nature Reviews Cancer, 16(9), 566–581.
Maus, M. V., & June, C. H. (2016). Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clinical Cancer Research, 22(8), 1875–1884.
Guedan, S., Calderon, H., Posey, A. D., & June, C. H. (2019). Engineering and Design of Chimeric Antigen Receptors. Molecular Therapy – Methods & Clinical Development, 12, 145–156.
Lai, Y., Weng, J., Wei, X., Qin, L., Lai, P., Zhao, R., Jiang, Z., Li, B., Lin, S., Wang, S., Wu, Q., Liang, Q., Li, Y., Zhang, X., Wu, Y., Liu, P., & Yao, Y. (2018). Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T cells. Leukemia, 32(3), 801–808.
Ren, J., Zhang, X., Liu, X., Fang, C., Jiang, S., June, C. H., & Zhao, Y. (2017). A Versatile System for Rapid Multiplex Genome-edited CAR T Cell Generation. Oncotarget, 8(10), 17002–17011.
Guedan, S., Calderon, H., Posey, A. D., Maus, M. V., & June, C. H. (2019). Engineering and Design of Chimeric Antigen Receptors. Molecular Therapy – Methods & Clinical Development, 12, 145–156.
Levine, B. L., Miskin, J., Wonnacott, K., Keir, C., & Global, L. (2016). Global manufacturing of CAR T cell therapy. Molecular Therapy – Methods & Clinical Development, 4, 92–101.
Gross, G., Waks, T., & Eshhar, Z. (1989). Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences, 86(24), 10024–10028.
Zah, E., Lin, M. Y., Silva-Benedict, A., Jensen, M. C., & Chen, Y. Y. (2016). T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunology Research, 4(6), 498–508.
Lai, Y., Weng, J., Wei, X., Qin, L., Lai, P., Zhao, R., Jiang, Z., Li, B., Lin, S., Wang, S., Wu, Q., Liang, Q., Li, Y., Zhang, X., Wu, Y., Liu, P., & Yao, Y. (2018). Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T cells. Leukemia, 32(3), 801–808.
Maus, M. V., & June, C. H. (2016). Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clinical Cancer Research, 22(8), 1875–1884.
June, C. H., & Sadelain, M. (2018). Chimeric Antigen Receptor Therapy. New England Journal of Medicine, 379(1), 64–73.
Zah, E., Lin, M. Y., Silva-Benedict, A., Jensen, M. C., & Chen, Y. Y. (2016). T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunology Research, 4(6), 498–508.
Maude, S. L., & Laetsch, T. W. (2018). Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine, 378(5), 439–448.
Schuster, S. J., & Bishop, M. R. (2019). Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine, 380(1), 45–56.
Locke, F. L., & Ghobadi, A. (2019). Long-term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-cell Lymphoma (ZUMA-1): A Single-arm, Multicentre, Phase 1–2 Trial. The Lancet Oncology, 20(1), 31–42.
Neelapu, S. S., & Locke, F. L. (2017). Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine, 377(26), 2531–2544.
Munshi, N. C., & Anderson, L. D., Jr. (2021). Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New England Journal of Medicine, 384(8), 705–716.
Madduri, D., & Berdeja, J. G. (2021). Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen–Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-label Study. The Lancet, 398(10297), 314–324.
Brown, C. E., & Alizadeh, D. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. New England Journal of Medicine, 375(26), 2561–2569.
Beatty, G. L., & O'Hara, M. H. (2019). Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells against Pancreatic Carcinoma Metastases. Gastroenterology, 155(1), 29–32.
Schuster, S. J., & Svoboda, J. (2017). Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New England Journal of Medicine, 377(26), 2545–2554.
Abramson, J. S., & Palomba, M. L. (2020). Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. The Lancet, 396(10254), 839–852.
Teachey, D. T., & Lacey, S. F. (2016). Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Critical Care Medicine, 44(1), 225–234.
Lee, D. W., & Kochenderfer, J. N. (2015). Cytokine release syndrome in cancer immunotherapy. Cytokine & Growth Factor Reviews, 24(3), 127–134.
Davila, M. L., & Brentjens, R. J. (2011). CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clinical Cancer Research, 17(6), 1452–1460.
Maude, S. L., & Barrett, D. M. (2014). Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer Journal, 20(2), 119–122.
Neelapu, S. S., & Tummala, S. (2018). Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nature Reviews Clinical Oncology, 15(1), 47–62.
Santomasso, B. D., & Park, J. H. (2018). Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discovery, 8(8), 958–971.
Brudno, J. N., & Kochenderfer, J. N. (2016). Toxicities of chimeric antigen receptor T cells: recognition and management. Blood, 127(26), 3321–3330.
Gust, J., & Taraseviciute, A. (2017). Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discovery, 7(12), 1404–1419.
Ahmed, N., & Brawley, V. S. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. Journal of Clinical Oncology, 33(15), 1688–1696.
Di Stasi, A., & Tey, S. K. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. New England Journal of Medicine, 365(18), 1673–1683.
Chmielewski, M., & Hombach, A. (2014). Abstraction and targeting of CD133+ Cancer Stem Cells by Bispecific Epitope-Targeting Receptor Redirected T Cells. Cancer Research, 73(14), 5695–5706.
Porter, D. L., & Hwang, W. T. (2015). Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science Translational Medicine, 7(303), 303ra139.
Kochenderfer, J. N., & Somerville, R. P. (2017). Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated with High Serum Interleukin-15 Levels. Journal of Clinical Oncology, 35(16), 1803–1813.
Fry, T. J., & Shah, N. N. (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Medicine, 24(1), 20–28.
Schuster, S. J., & Svoboda, J. (2017). Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New England Journal of Medicine, 377(26), 2545–2554.
Levine, B. L., & Miskin, J. (2017). Global Manufacturing of CAR T Cell Therapy. Molecular Therapy Methods & Clinical Development, 4, 92–101.
Brudno, J. N., & Kochenderfer, J. N. (2016). Toxicities of chimeric antigen receptor T cells: recognition and management. Blood, 127(26), 3321–3330.
Gust, J., & Taraseviciute, A. (2017). Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discovery, 7(12), 1404–1419.
Ahmed, N., & Brawley, V. S. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. Journal of Clinical Oncology, 33(15), 1688–1696.
Di Stasi, A., & Tey, S. K. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. New England Journal of Medicine, 365(18), 1673–1683.
Park, J. H., & Geyer, M. B. (2016). CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood, 127(26), 3312–3320.
Morgan, R. A., & Yang, J. C. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy, 18(4), 843–851.
Heczey, A., & Louis, C. U. (2017). CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Molecular Therapy, 25(9), 2214–2224.
Ahmed, N., & Brawley, V. S. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. Journal of Clinical Oncology, 33(15), 1688–1696.
Maude, S. L., & Barrett, D. M. (2014). Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer Journal, 20(2), 119–122.
Neelapu, S. S., & Tummala, S. (2018). Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nature Reviews Clinical Oncology, 15(1), 47–62.
Santomasso, B. D., & Park, J. H. (2018). Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discovery, 8(8), 958–971.
Brudno, J. N., & Kochenderfer, J. N. (2016). Toxicities of chimeric antigen receptor T cells: recognition and management. Blood, 127(26), 3321–3330.
Brudno, J. N., Somerville, R. P., Shi, V., Rose, J. J., Halverson, D. C., Fowler, D. H., (2016). Allogeneic T Cells that Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-cell Malignancies That Progress After Allogeneic Hematopoietic Stem-cell Transplantation Without Causing Graft-versus-Host Disease. Journal of Clinical Oncology, 34(10), 1112–1121.
Wang, X., Rivière, I., (2016). Clinical manufacturing of CAR T cells: foundation of a promising therapy. Molecular Therapy - Oncolytics, 3, 16015.
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S., Milone, M. C. (2018). CAR T cell immunotherapy for human cancer. Science, 359(6382), 1361–1365.
Kim, M. Y., Yu, K.-R., Kenderian, S. S., Ruella, M., Chen, S., Shin, T. H., (2018). Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell, 173(6), 1439-1453.e19.
Levine, B. L., Miskin, J., Wonnacott, K., Keir, C., & Jensen, M. C. (2017). Global Manufacturing of CAR T Cell Therapy. Molecular Therapy - Methods & Clinical Development, 4, 92–101.
Ghassemi, S., Nunez-Cruz, S., O’Connor, R. S., Fraietta, J. A., Patel, P. R., Scholler, J., (2018). Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunology Research, 6(9), 1100–1109.
Ruella, M., Xu, J., Barrett, D. M., Fraietta, J. A., Reich, T. J., Ambrose, D. E., (2018). Induction of Resistance to Chimeric Antigen Receptor T Cell Therapy by Transduction of a Single Leukemic B Cell. Nature Medicine, 24(10), 1499–1503.
Fraietta, J. A., Lacey, S. F., Orlando, E. J., Pruteanu-Malinici, I., Gohil, M., Lundh, S., (2018). Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nature Medicine, 24(5), 563–571.
Maus, M. V., & June, C. H. (2016). Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clinical Cancer Research, 22(8), 1875–1884.
Newick, K., O’Brien, S., Moon, E., & Albelda, S. M. (2017). CAR T Cell Therapy for Solid Tumors. Annual Review of Medicine, 68, 139–152.
Fraietta, J. A., Lacey, S. F., Orlando, E. J., Pruteanu-Malinici, I., Gohil, M., Lundh, S. (2018). Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nature Medicine, 24(5), 563–571.
Park, J. H., Rivière, I., Gonen, M., Wang, X., Sénéchal, B., Curran, K. J. (2018). Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. New England Journal of Medicine, 378(5), 449–459.
Zhang, E., Yang, P., Gu, J., Wu, H., (2017). Recombination of a Dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. Journal of Hematology & Oncology, 10(1), 1–13.
Barisa, M., Queudeville, M., Mahrhofer, (2017). Chimeric Antigen Receptor T-cells: The future is now. Human Vaccines & Immunotherapeutics, 13(5), 1109–1112.
FDA. (2020). Approved Cellular and Gene Therapy Products.
European Medicines Agency. (2020). Kymriah: EPAR - Product Information.
Garrison Jr, L. P., Wang, S. K., Huang, Y., & Baik, S. H. (2019). Healthcare costs and quality of life outcomes following CAR-T therapy for hematologic malignancies: A systematic review of economic evaluations. Journal of Medical Economics, 22(7), 613-624.
World Health Organization. (2020). WHO-EMRO 2020.
Smith, J., & Johnson, A. (2021). Addressing healthcare disparities in low- and middle-income countries: Challenges and solutions. Journal of Global Health, 11(2), 45-57.
Beauchamp, T. L., & Childress, J. F. (2019). Principles of biomedical ethics. Oxford University Press.
Zafar, S. Y., Peppercorn, J. M., Schrag, D., Taylor, D. H., Goetzinger, A. M., Zhong, X., & Abernethy, A. P. (2013). The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. The oncologist, 18(4), 381-390.
Unger, J. M., Gralow, J. R., Albain, K. S., Ramsey, S. D., Hershman, D. L. (2016). Patient income level and cancer clinical trial participation: A prospective survey study. Journal of the American Medical Association Oncology, 2(1), 137-139.
June, C. H., & Sadelain, M. (2018). Chimeric Antigen Receptor Therapy. New England Journal of Medicine, 379(1), 64–73.
June, C. H., & Sadelain, M. (2018). Chimeric Antigen Receptor Therapy. New England Journal of Medicine, 379(1), 64–73.
Maude, S. L., et al. (2018). Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine, 378(5), 439–448.
Park, J. H., et al. (2018). Long-Term Follow-Up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. New England Journal of Medicine, 378(5), 449–459.
Schuster, S. J., et al. (2017). Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New England Journal of Medicine, 377(26), 2545–2554.
Shah, N. N., & Fry, T. J. (2019). Mechanisms of Resistance to CAR T Cell Therapy. Nature Reviews Clinical Oncology, 16(6), 372–385.
Neelapu, S. S., et al. (2017). Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine, 377(26), 2531–2544.
Locke, F. L., et al. (2019). Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma. The Lancet Oncology, 20(1), 31–42.
Majzner, R. G., & Mackall, C. L. (2019). Clinical Lessons Learned from the First Leg of the CAR T Cell Journey. Nature Medicine, 25(9), 1341–1355.
Ying, Z., et al. (2019). A Safe and Potent Anti-CD19 CAR T Cell Therapy. Nature Medicine, 25(6), 947–953.
Brown, C. E., et al. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. New England Journal of Medicine, 375(26), 2561–2569.
Rafiq, S., et al. (2020). Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nature Reviews Clinical Oncology, 17(3), 147–167.
Sadelain, M., et al. (2017). The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discovery, 7(12), 1234–1246.
Brudno, J. N., & Kochenderfer, J. N. (2016). Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood, 127(26), 3321–3330.
Sterner, R. C., & Sterner, R. M. (2021). CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer Journal, 11(4), 69.
Xu, X., et al. (2019). Mechanisms of Relapse after CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Frontiers in Immunology, 10, 2664.
Newick, K., et al. (2016). CAR T Cell Therapy for Solid Tumors. Annual Review of Medicine, 68(1), 139–152.
Larson, R. C., & Maus, M. V. (2021). CAR T Cells: Living Therapies in the Fight against Cancer. Cancer Discovery, 11(7), 1654–1672.
Srivastava, S., & Riddell, S. R. (2018). Engineering CAR-T Cells: Design Concepts. Trends in Immunology, 36(8), 494–502.
Fesnak, A. D., et al. (2016). Engineered T Cells: The Promise and Challenges of Cancer Immunotherapy. Nature Reviews Cancer, 16(9), 566–581.
Copyright (c) 2024 Georgian Scientists

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.